FDA Warning Letters: Pet Food Manufacturer and Dairy Operation
On Jan. 2, 2015, FDA’s office in Philadelphia sent a warning letter to Nestle Purina PetCare noting that an inspection of the company’s low-acid canned food manufacturing facility in Allenton had been made from Sept. 15-Oct. 1, 2014.

The agency acknowledged receipt of a letter from the company stating that the processes had been evaluated and the products had “received a sufficient thermal process to achieve commercial sterility.”
However, FDA stated, the company’s response was not considered acceptable because no documentation was provided regarding evaluation of the entire product lot nor were any documentation or specifics provided of a corrective action plan to prevent such occurrences in the future.
Additional concerns cited in the FDA warning letter were that the pouch thickness for the company’s processed pet food products were not being monitored or documented as required by regulations, cooling water had not been adequately chlorinated or otherwise sanitized, and can conveyors and the reject chute did not have adequate protections in place to prevent an unprocessed can from falling into the cooling canal in the event of a can jam or other equipment malfunction.
FDA’s district office in Stoneham, MA, sent a letter dated Dec. 31, 2014, to Flood Brothers LLC in Clinton, ME, relating that inspections of the company’s dairy operation had been conducted on Nov. 4, Nov. 6, and Nov. 19, 2014. These inspections found violations of the Federal Food, Drug, and Cosmetic Act (FD&C Act), according to the agency’s letter.
Specifically, FDA alleged that Flood Brothers sold a dairy cow for slaughter as food in August 2014 which was later found to have flunixin at 0.253 ppm in its liver tissue. FDA has established a tolerance of 0.125 ppm for residues of flunixin in the liver of cattle, the letter stated.
“The presence of this drug in edible tissues from this animal in this amount causes the food to be adulterated” within the meaning of the FD&C Act, the agency stated, adding that the dairy had also failed to maintain complete treatment records.
In each letter, FDA requested that the companies provide written responses detailing steps taken to bring the facilities into compliance with food-safety laws and regulations, to correct violations cited in the letters, and to prevent their recurrence.
Recipients of these warning letters have 15 working days from receipt to outline specific steps they have taken to come into compliance with the law.

相关热词搜索:
[责任编辑:]

 Mettler-Toledo 在中国国际渔业博览会上展示创新的产品
Mettler-Toledo 在中国国际渔业博览会上展示创新的产品
 食品异物问题频发?是时候了解X射线检测了
食品异物问题频发?是时候了解X射线检测了
 开拓科技创新,撬动橡塑业高质量发展
开拓科技创新,撬动橡塑业高质量发展
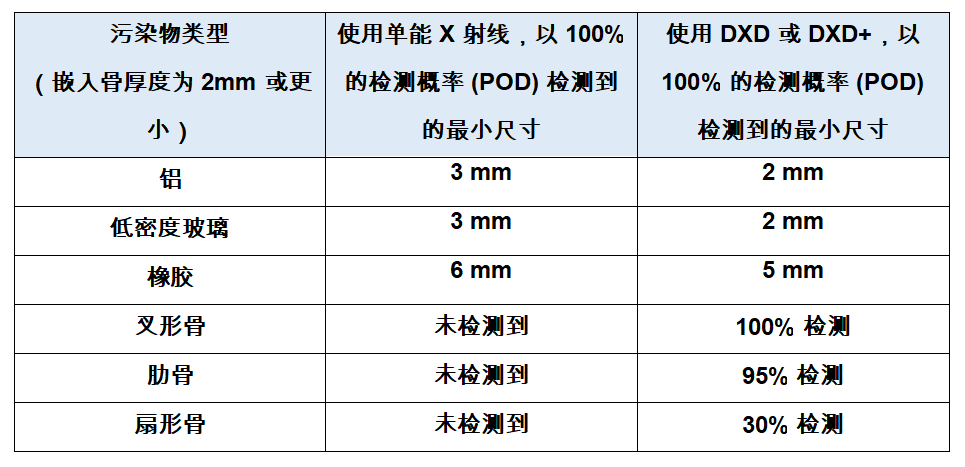 梅特勒托利多针对“难以发现”的污染物推出高品质X射线检
梅特勒托利多针对“难以发现”的污染物推出高品质X射线检
 探索婴幼儿辅食市场高质量发展之路,为宝宝成长保驾护航
探索婴幼儿辅食市场高质量发展之路,为宝宝成长保驾护航
 《食品安全最佳实践白皮书(2021-2022年)》四大主题发布
《食品安全最佳实践白皮书(2021-2022年)》四大主题发布
 《保健食品真实世界研究通则》团标技术审查与特食跨
《保健食品真实世界研究通则》团标技术审查与特食跨
 凝聚全球食饮智慧 SIAL西雅展国际化水平再创新高
凝聚全球食饮智慧 SIAL西雅展国际化水平再创新高
 精准把控 高质发展,第三届微生物安全与应用会议在
精准把控 高质发展,第三届微生物安全与应用会议在
 《食品行业科技创新白皮书》重磅发布!
《食品行业科技创新白皮书》重磅发布!






参与评论